6.1.1.2 Models of Atom Activity - Science 8
Storyboard Text
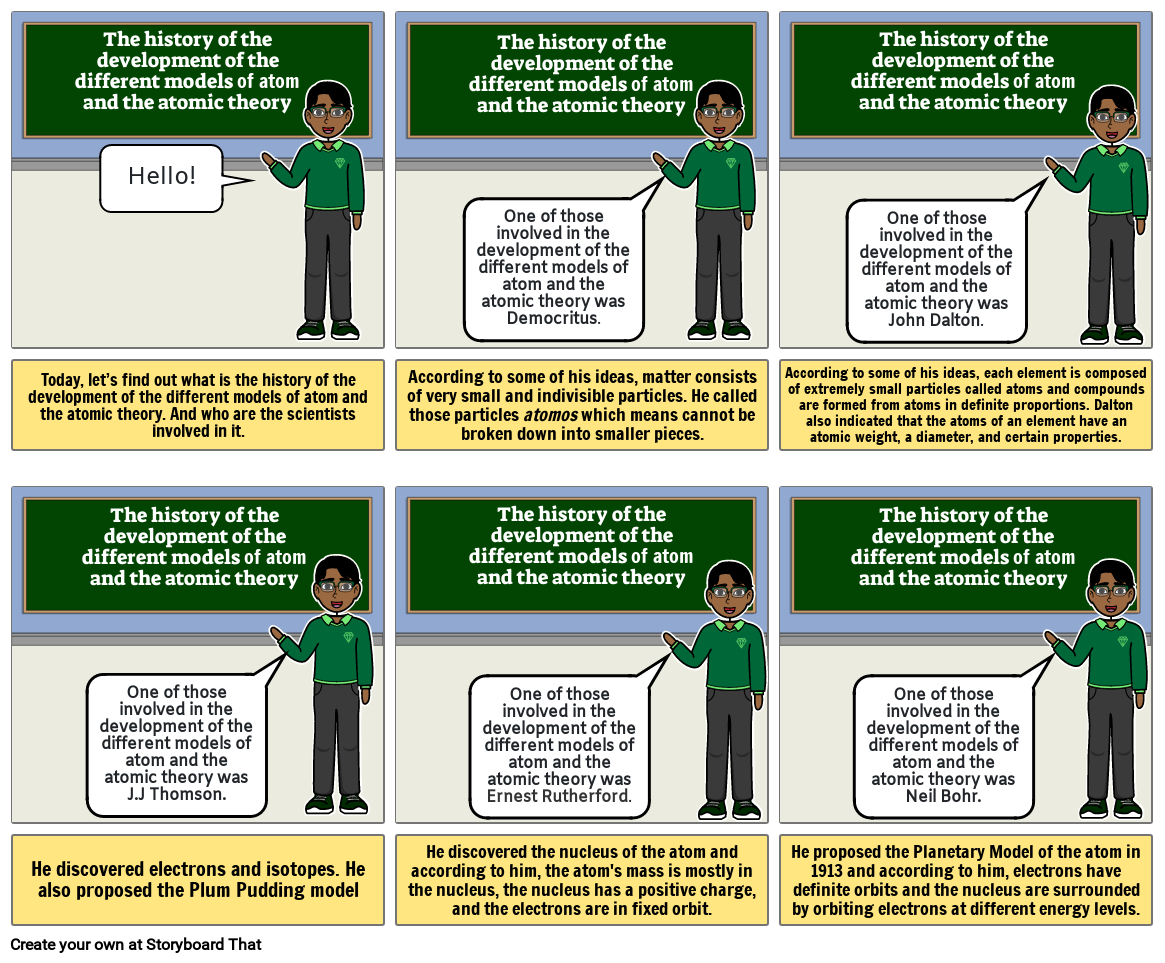
- The history of the development of the different models of atom and the atomic theory
- Hello!
- One of those involved in the development of the different models of atom and the atomic theory was Democritus.
- The history of the development of the different models of atom and the atomic theory
- The history of the development of the different models of atom and the atomic theory
- One of those involved in the development of the different models of atom and the atomic theory was John Dalton.
- Today, let’s find out what is the history of the development of the different models of atom and the atomic theory. And who are the scientists involved in it.
- The history of the development of the different models of atom and the atomic theory
- One of those involved in the development of the different models of atom and the atomic theory was J.J Thomson.
- According to some of his ideas, matter consists of very small and indivisible particles. He called those particles atomos which means cannot be broken down into smaller pieces.
- The history of the development of the different models of atom and the atomic theory
- One of those involved in the development of the different models of atom and the atomic theory wasErnest Rutherford.
- According to some of his ideas, each element is composed of extremely small particles called atoms and compounds are formed from atoms in definite proportions. Dalton also indicated that the atoms of an element have an atomic weight, a diameter, and certain properties.
- The history of the development of the different models of atom and the atomic theory
- One of those involved in the development of the different models of atom and the atomic theory was Neil Bohr.
- He discovered electrons and isotopes. He also proposed the Plum Pudding model
- He discovered the nucleus of the atom and according to him, the atom's mass is mostly in the nucleus, the nucleus has a positive charge, and the electrons are in fixed orbit.
- He proposed the Planetary Model of the atom in 1913 and according to him, electrons have definite orbits and the nucleus are surrounded by orbiting electrons at different energy levels.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
