Student Activities for Teaching Atoms
How Tos about Understanding Atomic Structures
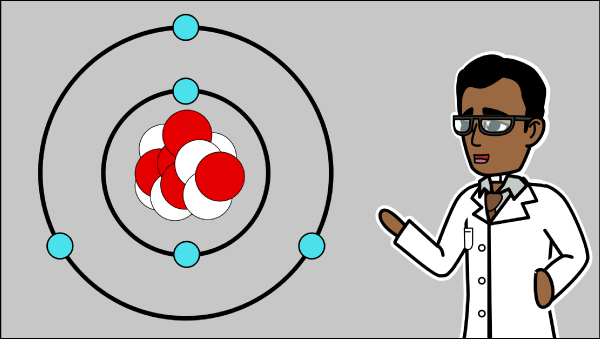
Spark curiosity with a hands-on atom model activity for your classroom
Engage students by letting them build simple atom models with everyday materials. Hands-on activities make abstract concepts concrete and memorable, helping learners visualize atomic structure in a fun, interactive way.
Gather easy-to-find materials for each student or group
Collect items like colored beads, clay, pipe cleaners, or buttons to represent protons, neutrons, and electrons. Accessible supplies make the activity simple to set up and inclusive for all students.
Guide students to build and label their own atom models
Show a real-world example and walk students through assembling their models. Encourage them to label each part—nucleus, electron cloud, protons, neutrons, electrons—for clear understanding of atomic structure.
Connect the activity to real-life objects and scientific concepts
Relate atom models to molecules in water, air, or classroom items. Making connections helps students see how atoms build everything around them, strengthening their grasp of chemistry and physics foundations.
Encourage reflection and classroom discussion
Ask students to share what surprised them and how their understanding changed. Reflection and discussion foster deeper learning and help you address misconceptions on atomic structure.
Frequently Asked Questions about Understanding Atomic Structures
What is an atom in simple terms?
An atom is the smallest unit of matter that makes up everything around us. Atoms combine to form molecules and substances we see and use every day.
How can I teach atomic structure to elementary students?
Use visual aids, analogies, and hands-on activities to help elementary students grasp atomic structure. Comparing atoms to building blocks or planets can make concepts more relatable and fun.
What are effective classroom activities for teaching atoms?
Model building, interactive diagrams, and storytelling are great activities for teaching atoms. These approaches help students visualize atomic parts and their roles.
Why is understanding atoms important in science education?
Understanding atoms is crucial because they are the foundation of chemistry and physics. A solid grasp of atomic concepts builds knowledge for future scientific learning.
What analogies help students visualize atoms?
Analogies like planets orbiting the sun or LEGO bricks building objects can help students picture atoms and their structure more easily.
- 1-3_Electron_orbitals • fickleandfreckled • License Attribution (http://creativecommons.org/licenses/by/2.0/)
- Cathode ray tube • Micah Sittig • License Attribution (http://creativecommons.org/licenses/by/2.0/)
- Democritus LACMA AC1993.213.2 • Fæ • License Attribution (http://creativecommons.org/licenses/by/2.0/)
- diffracted hydrogen • cosmiccandace • License Attribution (http://creativecommons.org/licenses/by/2.0/)
- HD.3A.002 • ENERGY.GOV • License United States Government Work (http://www.usa.gov/copyright.shtml)
- JOHN DALTON'S ATOMIC TABLES • summonedbyfells • License Attribution (http://creativecommons.org/licenses/by/2.0/)
- Portrait of Ernest Rutherford (1871-1937), Physicist and Chemist • Smithsonian Institution • License No known copyright restrictions (http://flickr.com/commons/usage/)
© 2025 - Clever Prototypes, LLC - All rights reserved.
StoryboardThat is a trademark of Clever Prototypes, LLC, and Registered in U.S. Patent and Trademark Office