atom pt1
Storyboard Text
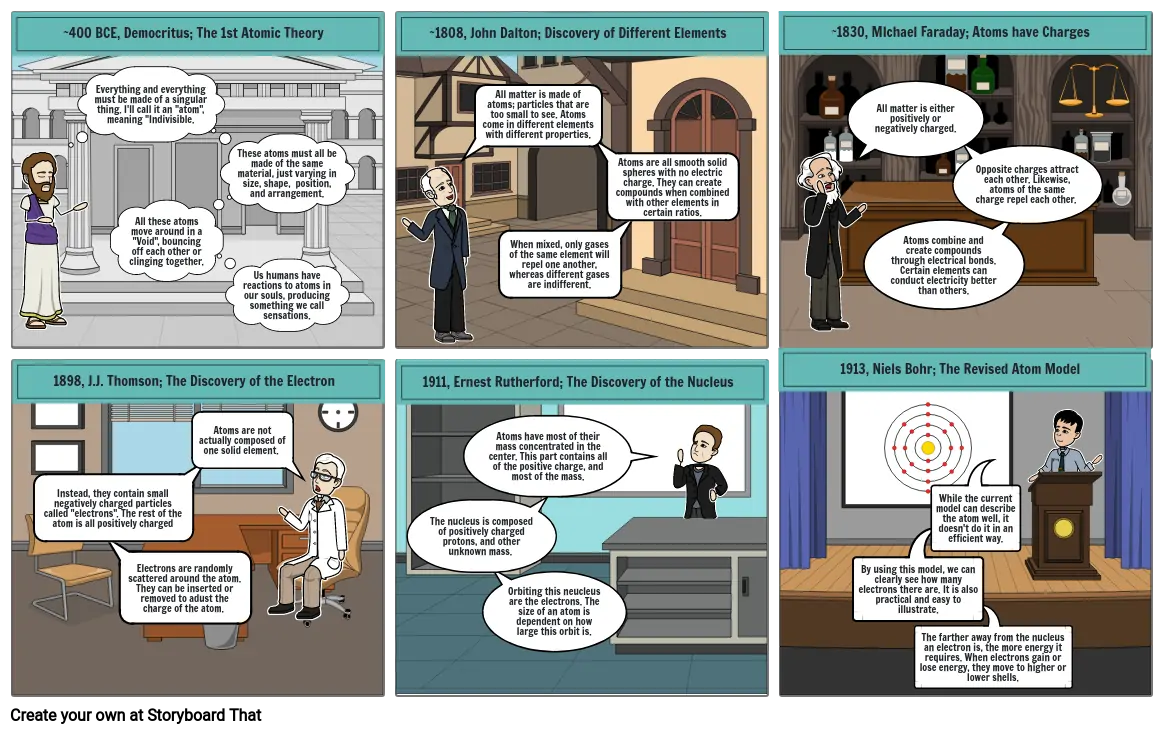
- ~400 BCE, Democritus; The 1st Atomic Theory
- Everything and everything must be made of a singular thing. I'll call it an "atom", meaning "Indivisible.
- All these atoms move around in a "Void", bouncing off each other or clinging together.
- These atoms must all be made of the same material, just varying in size, shape, position, and arrangement.
- Us humans have reactions to atoms in our souls, producing something we call sensations.
- ~1808, John Dalton; Discovery of Different Elements
- All matter is made of atoms; particles that are too small to see. Atoms come in different elements with different properties.
- When mixed, only gases of the same element will repel one another, whereas different gases are indifferent.
- Atoms are all smooth solid spheres with no electric charge. They can create compounds when combined with other elements in certain ratios.
- 1913, Niels Bohr; The Revised Atom Model
- ~1830, MIchael Faraday; Atoms have Charges
- All matter is either positively or negatively charged.
- Atoms combine and create compounds through electrical bonds. Certain elements can conduct electricity better than others.
- Opposite charges attract each other. Likewise, atoms of the same charge repel each other.
- 1898, J.J. Thomson; The Discovery of the Electron
- Instead, they contain small negatively charged particles called "electrons". The rest of the atom is all positively charged
- Electrons are randomly scattered around the atom. They can be inserted or removed to adust the charge of the atom.
- Atoms are not actually composed of one solid element.
- 1911, Ernest Rutherford; The Discovery of the Nucleus
- The nucleus is composed of positively charged protons, and other unknown mass.
- Orbiting this neucleus are the electrons. The size of an atom is dependent on how large this orbit is.
- Atoms have most of their mass concentrated in the center. This part contains all of the positive charge, and most of the mass.
- By using this model, we can clearly see how many electrons there are. It is also practical and easy to illustrate.
- While the current model can describe the atom well, it doesn't do it in an efficient way.
- The farther away from the nucleus an electron is, the more energy it requires. When electrons gain or lose energy, they move to higher or lower shells.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
