intermolecular forces
Storyboard Text
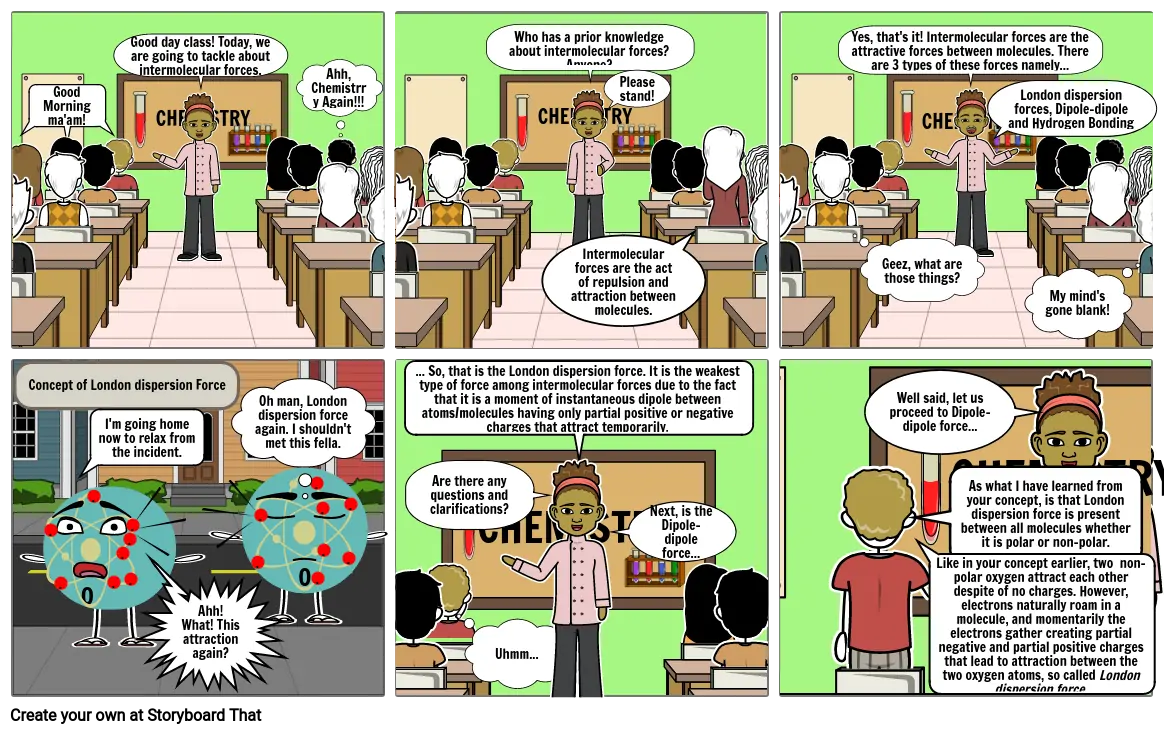
- Good Morning ma'am!
- Good day class! Today, we are going to tackle about intermolecular forces.
- CHEMISTRY
- Ahh, Chemistrry Again!!!
- Who has a prior knowledge about intermolecular forces? Anyone?
- CHEMISTRY
- Intermolecular forces are the act of repulsion and attraction between molecules.
- Please stand!
- Yes, that's it! Intermolecular forces are the attractive forces between molecules. There are 3 types of these forces namely...
- Geez, what are those things?
- CHEMISTRY
- London dispersion forces, Dipole-dipoleand Hydrogen Bonding
- My mind's gone blank!
- Concept of London dispersion Force
- O
- .
- .
- I'm going home now to relax from the incident.
- .
- .
- .
- .
- .
- .
- Ahh! What! This attraction again?
- Oh man, London dispersion force again. I shouldn't met this fella.
- O
- .
- .
- .
- .
- .
- .
- .
- .
- ... So, that is the London dispersion force. It is the weakest type of force among intermolecular forces due to the fact that it is a moment of instantaneous dipole between atoms/molecules having only partial positive or negative charges that attract temporarily.
- Uhmm...
- CHEMISTRY
- Are there any questions and clarifications?
- Next, is the Dipole-dipole force...
- As what I have learned from your concept, is that London dispersion force is present between all molecules whether it is polar or non-polar.
- Like in your concept earlier, two non-polar oxygen attract each other despite of no charges. However, electrons naturally roam in a molecule, and momentarily the electrons gather creating partial negative and partial positive charges that lead to attraction between the two oxygen atoms, so called London dispersion force.
- CHEMISTRY
- Well said, let us proceed to Dipole-dipole force...
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
