physics
Storyboard Text
- O
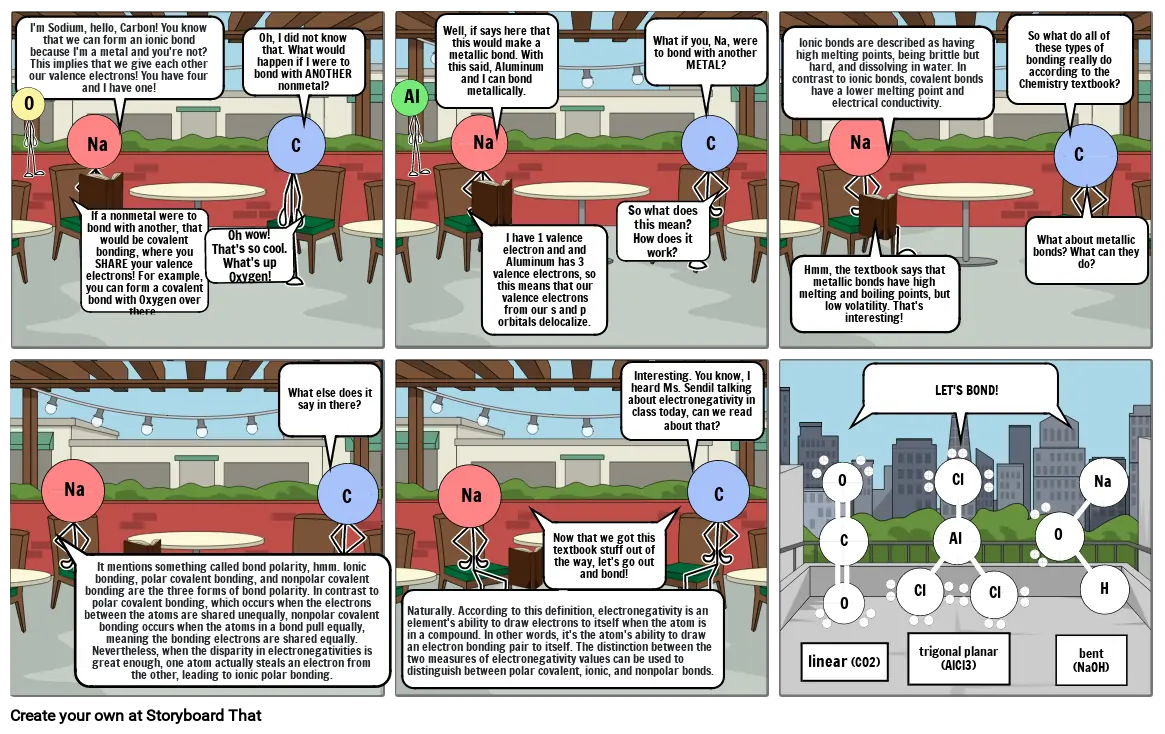
- I'm Sodium, hello, Carbon! You know that we can form an ionic bond because I'm a metal and you're not? This implies that we give each other our valence electrons! You have four and I have one!
- Na
- If a nonmetal were to bond with another, that would be covalent bonding, where you SHARE your valence electrons! For example, you can form a covalent bond with Oxygen over there.
- Oh wow! That's so cool. What's up Oxygen!
- Oh, I did not know that. What would happen if I were to bond with ANOTHER nonmetal?
- C
- Al
- Well, if says here that this would make a metallic bond. With this said, Aluminum and I can bond metallically.
- Na
- I have 1 valence electron and and Aluminum has 3 valence electrons, so this means that our valence electrons from our s and p orbitals delocalize.
- So what does this mean? How does it work?
- What if you, Na, were to bond with another METAL?
- C
- Ionic bonds are described as having high melting points, being brittle but hard, and dissolving in water. In contrast to ionic bonds, covalent bonds have a lower melting point and electrical conductivity.
- Hmm, the textbook says that metallic bonds have high melting and boiling points, but low volatility. That's interesting!
- Na
- LET'S BOND!
- So what do all of these types of bonding really do according to the Chemistry textbook?
- What about metallic bonds? What can they do?
- C
- Na
- It mentions something called bond polarity, hmm. Ionic bonding, polar covalent bonding, and nonpolar covalent bonding are the three forms of bond polarity. In contrast to polar covalent bonding, which occurs when the electrons between the atoms are shared unequally, nonpolar covalent bonding occurs when the atoms in a bond pull equally, meaning the bonding electrons are shared equally. Nevertheless, when the disparity in electronegativities is great enough, one atom actually steals an electron from the other, leading to ionic polar bonding.
- What else does it say in there?
- C
- Naturally. According to this definition, electronegativity is an element's ability to draw electrons to itself when the atom is in a compound. In other words, it's the atom's ability to draw an electron bonding pair to itself. The distinction between the two measures of electronegativity values can be used to distinguish between polar covalent, ionic, and nonpolar bonds.
- Na
- Now that we got this textbook stuff out of the way, let's go out and bond!
- Interesting. You know, I heard Ms. Sendil talking about electronegativity in class today, can we read about that?
- C
- O
- C
- O
- linear (CO2)
- Cl
- trigonal planar (AlCl3)
- Al
- Cl
- Cl
- O
- bent (NaOH)
- Na
- H
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
