Unknown Story
Storyboard Text
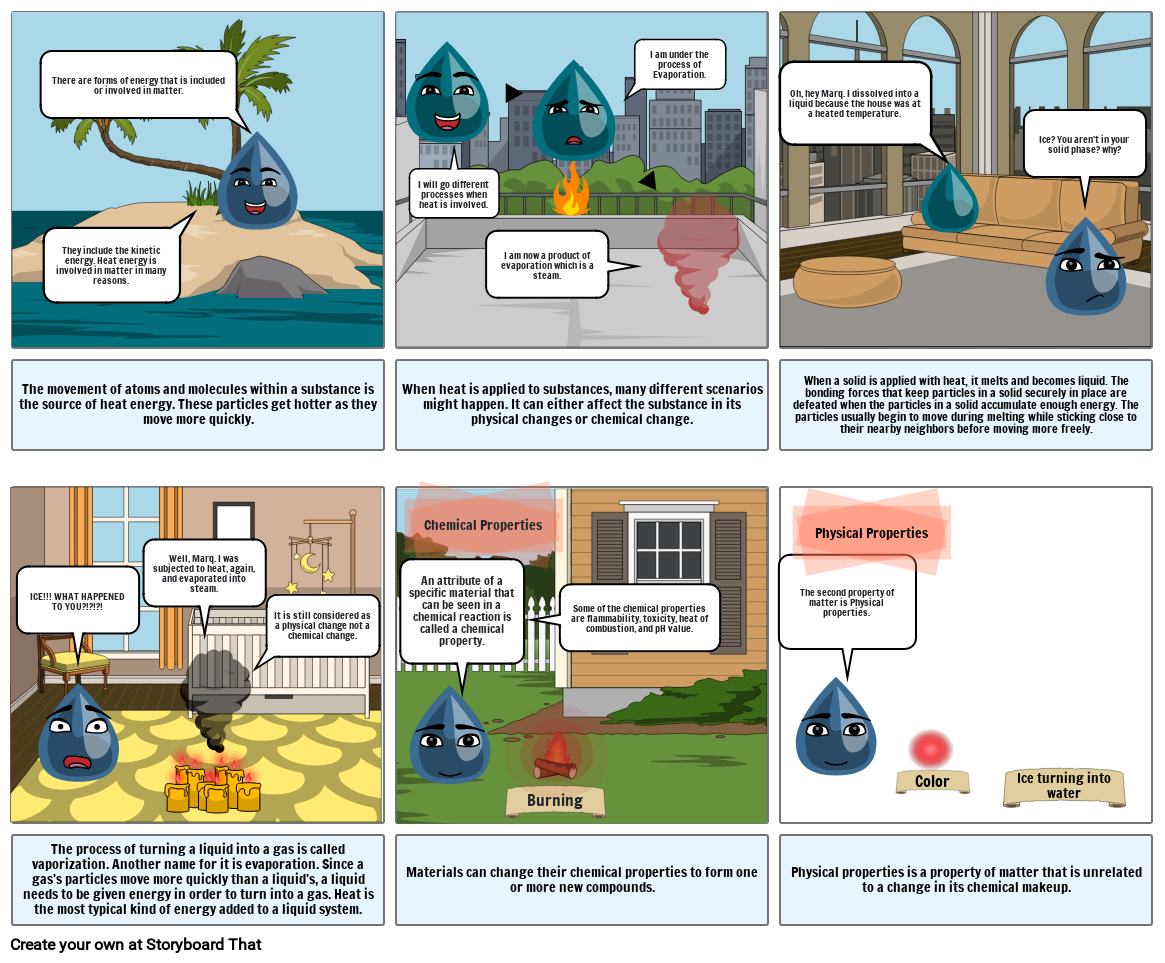
- There are forms of energy that is included or involved in matter.
- They include the kinetic energy. Heat energy is involved in matter in many reasons.
- I will go different processes when heat is involved.
- I am under the process of Evaporation.
- I am now a product of evaporation which is a steam.
- Oh, hey Marq. I dissolved into a liquid because the house was at a heated temperature.
- Ice? You aren't in your solid phase? why?
- The movement of atoms and molecules within a substance is the source of heat energy. These particles get hotter as they move more quickly.
- ICE!!! WHAT HAPPENED TO YOU?!?!?!
- Well, Marq. I was subjected to heat, again, and evaporated into steam.
- It is still considered as a physical change not a chemical change.
- When heat is applied to substances, many different scenarios might happen. It can either affect the substance in its physical changes or chemical change.
- An attribute of a specific material that can be seen in a chemical reaction is called a chemical property.
- Chemical Properties
- Some of the chemical properties are flammability, toxicity, heat of combustion, and pH value.
- The second property of matter is Physical properties.
- When a solid is applied with heat, it melts and becomes liquid. The bonding forces that keep particles in a solid securely in place are defeated when the particles in a solid accumulate enough energy. The particles usually begin to move during melting while sticking close to their nearby neighbors before moving more freely.
- Physical Properties
- The process of turning a liquid into a gas is called vaporization. Another name for it is evaporation. Since a gas's particles move more quickly than a liquid's, a liquid needs to be given energy in order to turn into a gas. Heat is the most typical kind of energy added to a liquid system.
- Materials can change their chemical properties to form one or more new compounds.
- Burning
- Physical properties is a property of matter that is unrelated to a change in its chemical makeup.
- Color
- Ice turning into water
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
