chem
Storyboard Text
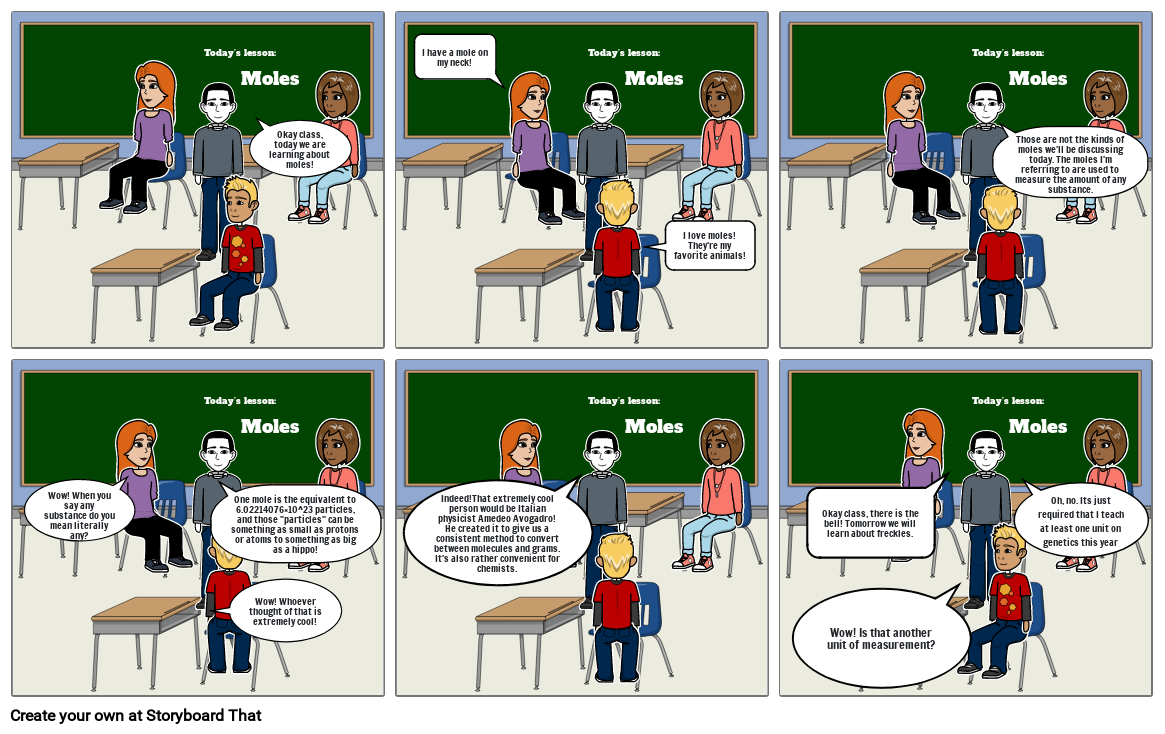
- Today's lesson:
- Moles
- Okay class, today we are learning about moles!
- I have a mole on my neck!
- Today's lesson:
- Moles
- I love moles! They're my favorite animals!
- Today's lesson:
- Moles
- Those are not the kinds of moles we'll be discussing today. The moles I'm referring to are used to measure the amount of any substance.
- Wow! When you say any substance do you mean literally any?
- Today's lesson:
- Wow! Whoever thought of that is extremely cool!
- One mole is the equivalent to 6.02214076×10^23 particles, and those "particles" can be something as small as protons or atoms to something as big as a hippo!
- Moles
- Indeed!That extremely cool person would be Italian physicist Amedeo Avogadro! He created it to give us a consistent method to convert between molecules and grams. It's also rather convenient for chemists.
- Today's lesson:
- Moles
- Wow! Is that another unit of measurement?
- Okay class, there is the bell! Tomorrow we will learn about freckles.
- Today's lesson:
- Moles
- Oh, no. Its just required that I teach at least one unit on genetics this year
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!


