Story Board-Chemical properties of oxygen gas-Vagdevi
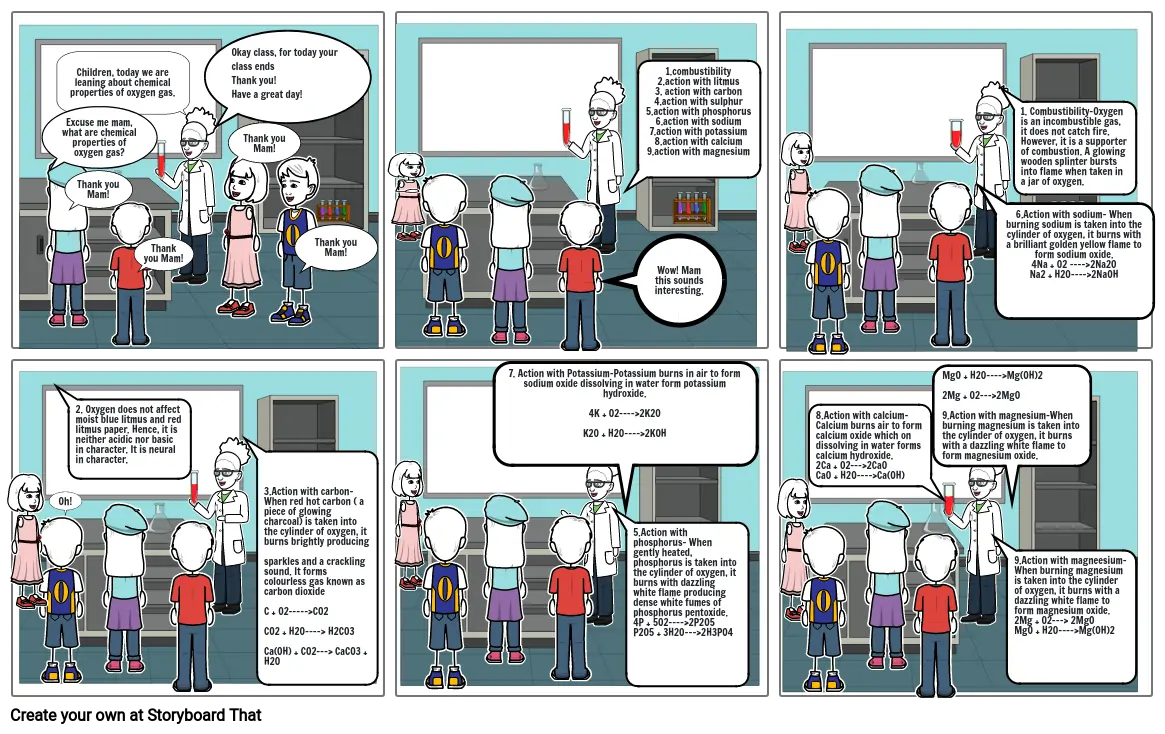
Children, today we are leaning about chemical properties of oxygen gas.
Excuse me mam, what are chemical properties of oxygen gas?
1.combustibility
2.action with litmus
3. action with carbon
4.action with sulphur
5.action with phosphorus
6.action with sodium
7.action with potassium
8.action with calcium
9.action with magnesium
Wow! Mam this sounds interesting.
1. Combustibility-Oxygen is an incombustible gas, it does not catch fire. However, it is a supporter of combustion. A glowing wooden splinter bursts into flame when taken in a jar of oxygen.
2. Oxygen does not affect moist blue litmus and red litmus paper. Hence, it is neither acidic nor basic in character. It is neural in character.
Oh!
5.Action with phosphorus- When gently heated, phosphorus is taken into the cylinder of oxygen, it burns with dazzling white flame producing dense white fumes of phosphorus pentoxide.
4P + 5O2----2P2O5
P2O5 + 3H2O---2H3PO4
9.Action with magneesium-
When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
2Mg + O2--- 2MgO
MgO + H2O----Mg(OH)2
MgO + H2O----Mg(OH)2
2Mg + O2---2MgO
9.Action with magnesium-When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
7. Action with Potassium-Potassium burns in air to form sodium oxide dissolving in water form potassium hydroxide.
4K + O2----2K2O
K2O + H2O----2KOH
3.Action with carbon- When red hot carbon ( a piece of glowing charcoal) is taken into the cylinder of oxygen, it burns brightly producing
sparkles and a crackling sound. It forms colourless gas known as carbon dioxide
C + O2-----CO2
CO2 + H2O---- H2CO3
Ca(OH) + CO2--- CaCO3 + H2O
8.Action with calcium- Calcium burns air to form calcium oxide which on dissolving in water forms calcium hydroxide.
2Ca + O2---2CaO
CaO + H2O----Ca(OH)
6.Action with sodium- When burning sodium is taken into the cylinder of oxygen, it burns with a brilliant golden yellow flame to form sodium oxide.
4Na + O2 ----2Na2O
Na2 + H2O----2NaOH
Thank you Mam!
Thank you Mam!
Thank you Mam!
Thank you Mam!
Children, today we are leaning about chemical properties of oxygen gas.
Excuse me mam, what are chemical properties of oxygen gas?
1.combustibility
2.action with litmus
3. action with carbon
4.action with sulphur
5.action with phosphorus
6.action with sodium
7.action with potassium
8.action with calcium
9.action with magnesium
Wow! Mam this sounds interesting.
1. Combustibility-Oxygen is an incombustible gas, it does not catch fire. However, it is a supporter of combustion. A glowing wooden splinter bursts into flame when taken in a jar of oxygen.
2. Oxygen does not affect moist blue litmus and red litmus paper. Hence, it is neither acidic nor basic in character. It is neural in character.
Oh!
5.Action with phosphorus- When gently heated, phosphorus is taken into the cylinder of oxygen, it burns with dazzling white flame producing dense white fumes of phosphorus pentoxide.
4P + 5O2----2P2O5
P2O5 + 3H2O---2H3PO4
9.Action with magneesium-
When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
2Mg + O2--- 2MgO
MgO + H2O----Mg(OH)2
MgO + H2O----Mg(OH)2
2Mg + O2---2MgO
9.Action with magnesium-When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
7. Action with Potassium-Potassium burns in air to form sodium oxide dissolving in water form potassium hydroxide.
4K + O2----2K2O
K2O + H2O----2KOH
3.Action with carbon- When red hot carbon ( a piece of glowing charcoal) is taken into the cylinder of oxygen, it burns brightly producing
sparkles and a crackling sound. It forms colourless gas known as carbon dioxide
C + O2-----CO2
CO2 + H2O---- H2CO3
Ca(OH) + CO2--- CaCO3 + H2O
8.Action with calcium- Calcium burns air to form calcium oxide which on dissolving in water forms calcium hydroxide.
2Ca + O2---2CaO
CaO + H2O----Ca(OH)
6.Action with sodium- When burning sodium is taken into the cylinder of oxygen, it burns with a brilliant golden yellow flame to form sodium oxide.
4Na + O2 ----2Na2O
Na2 + H2O----2NaOH
Thank you Mam!
Thank you Mam!
Thank you Mam!
Thank you Mam!
Children, today we are leaning about chemical properties of oxygen gas.
Excuse me mam, what are chemical properties of oxygen gas?
1.combustibility
2.action with litmus
3. action with carbon
4.action with sulphur
5.action with phosphorus
6.action with sodium
7.action with potassium
8.action with calcium
9.action with magnesium
Wow! Mam this sounds interesting.
1. Combustibility-Oxygen is an incombustible gas, it does not catch fire. However, it is a supporter of combustion. A glowing wooden splinter bursts into flame when taken in a jar of oxygen.
2. Oxygen does not affect moist blue litmus and red litmus paper. Hence, it is neither acidic nor basic in character. It is neural in character.
Oh!
5.Action with phosphorus- When gently heated, phosphorus is taken into the cylinder of oxygen, it burns with dazzling white flame producing dense white fumes of phosphorus pentoxide.
4P + 5O2----2P2O5
P2O5 + 3H2O---2H3PO4
9.Action with magneesium-
When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
2Mg + O2--- 2MgO
MgO + H2O----Mg(OH)2
MgO + H2O----Mg(OH)2
2Mg + O2---2MgO
9.Action with magnesium-When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
7. Action with Potassium-Potassium burns in air to form sodium oxide dissolving in water form potassium hydroxide.
4K + O2----2K2O
K2O + H2O----2KOH
3.Action with carbon- When red hot carbon ( a piece of glowing charcoal) is taken into the cylinder of oxygen, it burns brightly producing
sparkles and a crackling sound. It forms colourless gas known as carbon dioxide
C + O2-----CO2
CO2 + H2O---- H2CO3
Ca(OH) + CO2--- CaCO3 + H2O
8.Action with calcium- Calcium burns air to form calcium oxide which on dissolving in water forms calcium hydroxide.
2Ca + O2---2CaO
CaO + H2O----Ca(OH)
6.Action with sodium- When burning sodium is taken into the cylinder of oxygen, it burns with a brilliant golden yellow flame to form sodium oxide.
4Na + O2 ----2Na2O
Na2 + H2O----2NaOH
Thank you Mam!
Thank you Mam!
Thank you Mam!
Thank you Mam!
Children, today we are leaning about chemical properties of oxygen gas.
Excuse me mam, what are chemical properties of oxygen gas?
1.combustibility
2.action with litmus
3. action with carbon
4.action with sulphur
5.action with phosphorus
6.action with sodium
7.action with potassium
8.action with calcium
9.action with magnesium
Wow! Mam this sounds interesting.
1. Combustibility-Oxygen is an incombustible gas, it does not catch fire. However, it is a supporter of combustion. A glowing wooden splinter bursts into flame when taken in a jar of oxygen.
2. Oxygen does not affect moist blue litmus and red litmus paper. Hence, it is neither acidic nor basic in character. It is neural in character.
Oh!
5.Action with phosphorus- When gently heated, phosphorus is taken into the cylinder of oxygen, it burns with dazzling white flame producing dense white fumes of phosphorus pentoxide.
4P + 5O2----2P2O5
P2O5 + 3H2O---2H3PO4
9.Action with magneesium-
When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
2Mg + O2--- 2MgO
MgO + H2O----Mg(OH)2
MgO + H2O----Mg(OH)2
2Mg + O2---2MgO
9.Action with magnesium-When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
7. Action with Potassium-Potassium burns in air to form sodium oxide dissolving in water form potassium hydroxide.
4K + O2----2K2O
K2O + H2O----2KOH
3.Action with carbon- When red hot carbon ( a piece of glowing charcoal) is taken into the cylinder of oxygen, it burns brightly producing
sparkles and a crackling sound. It forms colourless gas known as carbon dioxide
C + O2-----CO2
CO2 + H2O---- H2CO3
Ca(OH) + CO2--- CaCO3 + H2O
8.Action with calcium- Calcium burns air to form calcium oxide which on dissolving in water forms calcium hydroxide.
2Ca + O2---2CaO
CaO + H2O----Ca(OH)
6.Action with sodium- When burning sodium is taken into the cylinder of oxygen, it burns with a brilliant golden yellow flame to form sodium oxide.
4Na + O2 ----2Na2O
Na2 + H2O----2NaOH
Thank you Mam!
Thank you Mam!
Thank you Mam!
Thank you Mam!
Children, today we are leaning about chemical properties of oxygen gas.
Excuse me mam, what are chemical properties of oxygen gas?
1.combustibility
2.action with litmus
3. action with carbon
4.action with sulphur
5.action with phosphorus
6.action with sodium
7.action with potassium
8.action with calcium
9.action with magnesium
Wow! Mam this sounds interesting.
1. Combustibility-Oxygen is an incombustible gas, it does not catch fire. However, it is a supporter of combustion. A glowing wooden splinter bursts into flame when taken in a jar of oxygen.
2. Oxygen does not affect moist blue litmus and red litmus paper. Hence, it is neither acidic nor basic in character. It is neural in character.
Oh!
5.Action with phosphorus- When gently heated, phosphorus is taken into the cylinder of oxygen, it burns with dazzling white flame producing dense white fumes of phosphorus pentoxide.
4P + 5O2----2P2O5
P2O5 + 3H2O---2H3PO4
9.Action with magneesium-
When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
2Mg + O2--- 2MgO
MgO + H2O----Mg(OH)2
MgO + H2O----Mg(OH)2
2Mg + O2---2MgO
9.Action with magnesium-When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
7. Action with Potassium-Potassium burns in air to form sodium oxide dissolving in water form potassium hydroxide.
4K + O2----2K2O
K2O + H2O----2KOH
3.Action with carbon- When red hot carbon ( a piece of glowing charcoal) is taken into the cylinder of oxygen, it burns brightly producing
sparkles and a crackling sound. It forms colourless gas known as carbon dioxide
C + O2-----CO2
CO2 + H2O---- H2CO3
Ca(OH) + CO2--- CaCO3 + H2O
8.Action with calcium- Calcium burns air to form calcium oxide which on dissolving in water forms calcium hydroxide.
2Ca + O2---2CaO
CaO + H2O----Ca(OH)
6.Action with sodium- When burning sodium is taken into the cylinder of oxygen, it burns with a brilliant golden yellow flame to form sodium oxide.
4Na + O2 ----2Na2O
Na2 + H2O----2NaOH
Thank you Mam!
Thank you Mam!
Thank you Mam!
Thank you Mam!
Children, today we are leaning about chemical properties of oxygen gas.
Excuse me mam, what are chemical properties of oxygen gas?
1.combustibility
2.action with litmus
3. action with carbon
4.action with sulphur
5.action with phosphorus
6.action with sodium
7.action with potassium
8.action with calcium
9.action with magnesium
Wow! Mam this sounds interesting.
1. Combustibility-Oxygen is an incombustible gas, it does not catch fire. However, it is a supporter of combustion. A glowing wooden splinter bursts into flame when taken in a jar of oxygen.
2. Oxygen does not affect moist blue litmus and red litmus paper. Hence, it is neither acidic nor basic in character. It is neural in character.
Oh!
5.Action with phosphorus- When gently heated, phosphorus is taken into the cylinder of oxygen, it burns with dazzling white flame producing dense white fumes of phosphorus pentoxide.
4P + 5O2----2P2O5
P2O5 + 3H2O---2H3PO4
9.Action with magneesium-
When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
2Mg + O2--- 2MgO
MgO + H2O----Mg(OH)2
MgO + H2O----Mg(OH)2
2Mg + O2---2MgO
9.Action with magnesium-When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
7. Action with Potassium-Potassium burns in air to form sodium oxide dissolving in water form potassium hydroxide.
4K + O2----2K2O
K2O + H2O----2KOH
3.Action with carbon- When red hot carbon ( a piece of glowing charcoal) is taken into the cylinder of oxygen, it burns brightly producing
sparkles and a crackling sound. It forms colourless gas known as carbon dioxide
C + O2-----CO2
CO2 + H2O---- H2CO3
Ca(OH) + CO2--- CaCO3 + H2O
8.Action with calcium- Calcium burns air to form calcium oxide which on dissolving in water forms calcium hydroxide.
2Ca + O2---2CaO
CaO + H2O----Ca(OH)
6.Action with sodium- When burning sodium is taken into the cylinder of oxygen, it burns with a brilliant golden yellow flame to form sodium oxide.
4Na + O2 ----2Na2O
Na2 + H2O----2NaOH
Thank you Mam!
Thank you Mam!
Thank you Mam!
Thank you Mam!
Children, today we are leaning about chemical properties of oxygen gas.
Excuse me mam, what are chemical properties of oxygen gas?
1.combustibility
2.action with litmus
3. action with carbon
4.action with sulphur
5.action with phosphorus
6.action with sodium
7.action with potassium
8.action with calcium
9.action with magnesium
Wow! Mam this sounds interesting.
1. Combustibility-Oxygen is an incombustible gas, it does not catch fire. However, it is a supporter of combustion. A glowing wooden splinter bursts into flame when taken in a jar of oxygen.
2. Oxygen does not affect moist blue litmus and red litmus paper. Hence, it is neither acidic nor basic in character. It is neural in character.
Oh!
5.Action with phosphorus- When gently heated, phosphorus is taken into the cylinder of oxygen, it burns with dazzling white flame producing dense white fumes of phosphorus pentoxide.
4P + 5O2----2P2O5
P2O5 + 3H2O---2H3PO4
9.Action with magneesium-
When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
2Mg + O2--- 2MgO
MgO + H2O----Mg(OH)2
MgO + H2O----Mg(OH)2
2Mg + O2---2MgO
9.Action with magnesium-When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
7. Action with Potassium-Potassium burns in air to form sodium oxide dissolving in water form potassium hydroxide.
4K + O2----2K2O
K2O + H2O----2KOH
3.Action with carbon- When red hot carbon ( a piece of glowing charcoal) is taken into the cylinder of oxygen, it burns brightly producing
sparkles and a crackling sound. It forms colourless gas known as carbon dioxide
C + O2-----CO2
CO2 + H2O---- H2CO3
Ca(OH) + CO2--- CaCO3 + H2O
8.Action with calcium- Calcium burns air to form calcium oxide which on dissolving in water forms calcium hydroxide.
2Ca + O2---2CaO
CaO + H2O----Ca(OH)
6.Action with sodium- When burning sodium is taken into the cylinder of oxygen, it burns with a brilliant golden yellow flame to form sodium oxide.
4Na + O2 ----2Na2O
Na2 + H2O----2NaOH
Thank you Mam!
Thank you Mam!
Thank you Mam!
Thank you Mam!
Storyboard Text
- Excuse me mam, what are chemical properties of oxygen gas?
- Children, today we are leaning about chemical properties of oxygen gas.
- Thank you Mam!
- Thank you Mam!
- Okay class, for today your class endsThank you!Have a great day!
- Thank you Mam!
- Thank you Mam!
- Wow! Mam this sounds interesting.
- 1.combustibility2.action with litmus3. action with carbon4.action with sulphur5.action with phosphorus6.action with sodium7.action with potassium8.action with calcium9.action with magnesium
- 6.Action with sodium- When burning sodium is taken into the cylinder of oxygen, it burns with a brilliant golden yellow flame to form sodium oxide.4Na + O2 ---->2Na2ONa2 + H2O---->2NaOH
- 1. Combustibility-Oxygen is an incombustible gas, it does not catch fire. However, it is a supporter of combustion. A glowing wooden splinter bursts into flame when taken in a jar of oxygen.
- Oh!
- 2. Oxygen does not affect moist blue litmus and red litmus paper. Hence, it is neither acidic nor basic in character. It is neural in character.
- 3.Action with carbon- When red hot carbon ( a piece of glowing charcoal) is taken into the cylinder of oxygen, it burns brightly producingsparkles and a crackling sound. It forms colourless gas known as carbon dioxideC + O2----->CO2CO2 + H2O----> H2CO3Ca(OH) + CO2---> CaCO3 + H2O
- 7. Action with Potassium-Potassium burns in air to form sodium oxide dissolving in water form potassium hydroxide.4K + O2---->2K2OK2O + H2O---->2KOH
- 5.Action with phosphorus- When gently heated, phosphorus is taken into the cylinder of oxygen, it burns with dazzling white flame producing dense white fumes of phosphorus pentoxide.4P + 5O2---->2P2O5P2O5 + 3H2O--->2H3PO4
- 8.Action with calcium- Calcium burns air to form calcium oxide which on dissolving in water forms calcium hydroxide.2Ca + O2--->2CaOCaO + H2O---->Ca(OH)
- MgO + H2O---->Mg(OH)22Mg + O2--->2MgO9.Action with magnesium-When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.
- 9.Action with magneesium-When burning magnesium is taken into the cylinder of oxygen, it burns with a dazzling white flame to form magnesium oxide.2Mg + O2---> 2MgOMgO + H2O---->Mg(OH)2


