Unknown Story
Storyboard Text
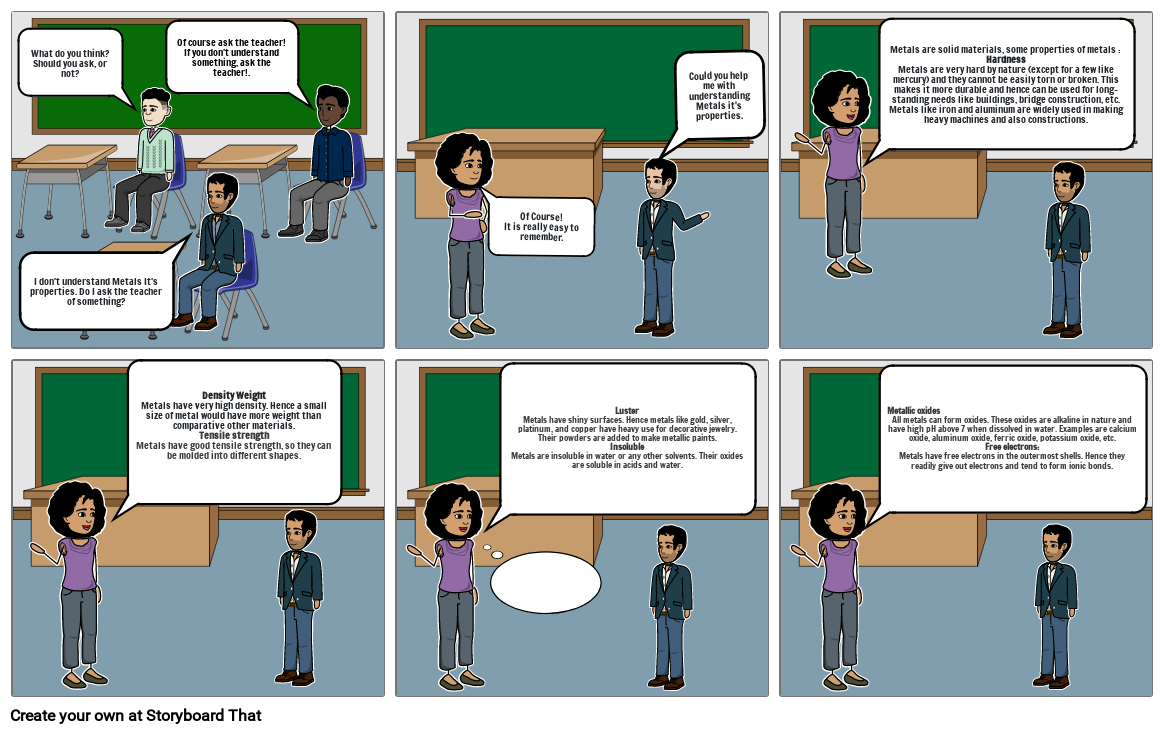
- What do you think? Should you ask, or not?
- I don't understand Metals It's properties. Do I ask the teacher of something?
- Of course ask the teacher! If you don't understand something, ask the teacher!.
- Of Course!It is really easy to remember.
- Could you help me with understanding Metals it's properties.
- Metals are solid materials, some properties of metals : HardnessMetals are very hard by nature (except for a few like mercury) and they cannot be easily torn or broken. This makes it more durable and hence can be used for long-standing needs like buildings, bridge construction, etc. Metals like iron and aluminum are widely used in making heavy machines and also constructions.
- Density WeightMetals have very high density. Hence a small size of metal would have more weight than comparative other materials.Tensile strengthMetals have good tensile strength, so they can be molded into different shapes.
- LusterMetals have shiny surfaces. Hence metals like gold, silver, platinum, and copper have heavy use for decorative jewelry. Their powders are added to make metallic paints.InsolubleMetals are insoluble in water or any other solvents. Their oxides are soluble in acids and water.
- Metallic oxidesAll metals can form oxides. These oxides are alkaline in nature and have high pH above 7 when dissolved in water. Examples are calcium oxide, aluminum oxide, ferric oxide, potassium oxide, etc.Free electrons:Metals have free electrons in the outermost shells. Hence they readily give out electrons and tend to form ionic bonds.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
